Pharmaceuticals & Research Reagents and Kits
Bacterial Endotoxin Assays – LAL Reagents
LAL Reagents
Introduction to the LAL Test
Limulus Amebocyte Lysate (LAL) tests detect and quantify bacterial endotoxins extracted from the outer membrane of gram negative bacteria. The critical component of the LAL reagents used in endotoxin tests is derived from blood cells (amebocytes) of the horseshoe crab, Limulus polyphemus. It contains the proteins of the blood clotting mechanism, which is triggered by endotoxins. LAL reagents are primarily used to test for endotoxins in injectable pharmaceuticals, biological products, and medical devices. They are also used in renal dialysis centers and a wide range of other applications. LAL tests are described in the Bacterial Endotoxins Test chapter in the United States Pharmacopeia (Chapter <85>) and in the equivalent chapters in the European Pharmacopoeia (Chapter 2.6.14) and the Japanese Pharmacopoeia (General Tests, No. 4.01).
LAL Test Methods
There are three principal LAL test methods: the gel-clot, turbidimetric and chromogenic methods. The latter two may be grouped together as photometric methods as they require an optical reader.
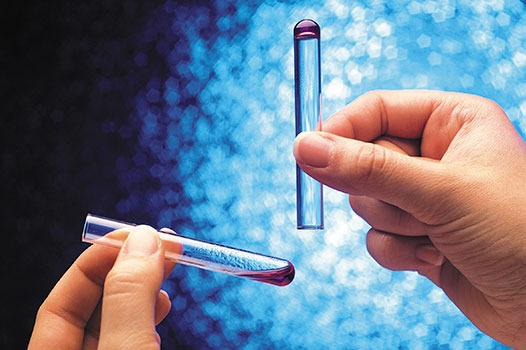
Gel-clot Method
This is the simplest LAL test. The formation of a gel-clot indicates the presence of endotoxin in a sample. The method is performed in small test tubes and is read manually by inverting the test tubes. The maximum sensitivity is 0.03 EU/mL.
- Requires non-circulating water bath or dry bath incubator
- Manually read test
- Reagents of differing sensitivity available: 0.5 (STV only), 0.25, 0.125, 0.06 and 0.03 EU/mL
- May be less sensitive to interference than other LAL methods
- Is the "gold standard" for the great majority of products in the United States, European and Japanese pharmacopeia (i.e. it is the default method used to resolve a dispute)
Turbidimetric Method
The optical density (turbidity) increase that accompanies the clotting reaction is read in the Pyros Kinetix® Flex tube reader or in a incubating microplate reader. The maximum sensitivity of 0.001 EU/mL can be achieved when uising ACC's Pyrotell®-T reagent in our Pyros Kinetix® or Pyros Kinetix® Flex tube reader.
- Requires the Pyros Kinetix® Flex tube reader system or an incubating microplate reader
- Maximum sensitivity to 0.001 EU/mL, highest level of sensitivity available in the LAL industry
- Incubation time varies depending on the standard curve range
- High sensitivity allows for greater dilution to overcome interference
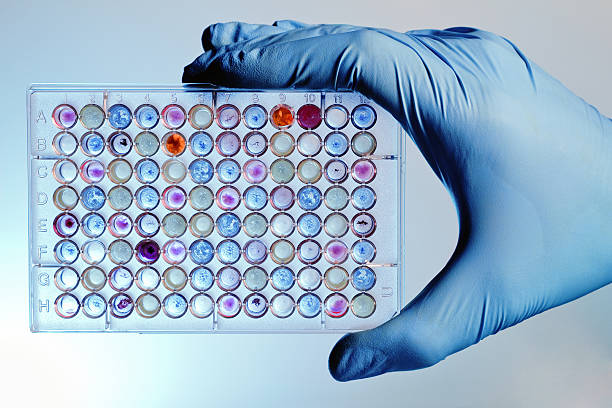
Chromogenic Method
The LAL reagent is formulated with a synthetic substrate which produces a chromophore when cleaved by endotoxin activated enzyme. The test is read at 405 nm, usually in a microplate reader. The maximum sensitivity of 0.001 EU/mL can be achieved when using ACC's Pyrochrome® Chromogenic reagent with Glucashield® Buffer.
- Requires a tube reader or incubating microplate reader (an incubating reader is required for the kinetic method)
- Maximum sensitivity to 0.001 EU/mL when using ACC's Pyrochrome® reagent with Glucashield® Buffer
- A quantitative assay providing electronic stored data and print-outs of results
- Incubation time varies depending on the standard curve range
- Higher sensitivity allows for greater dilution to overcome interference
- The option of a diazo-coupled endpoint method (read at 540–550 nm) is available, which is useful for samples that absorb light at 405 nm
More products
Pharmaceuticals & Research
Glucatell® Kit
The Glucatell® assay kit is specific for (1→3)-β-D-glucan. The assay is based...
Pharmaceuticals & Research
PyroSmart NextGen
PyroSmart NextGen® recombinant Cascade Reagent (rCR)...
Pharmaceuticals & Research
Gen5™ Secure Software
Designed by engineers focused on microplate instrument technology...
Pharmaceuticals & Research
BioTek Epoch 2 Microplate Spectrophotometer
The BioTek Epoch 2 microplate...
our foreign associates
Exclusive foreign partnerships
partner to success
Expand Your Reach In India With KHC. You Will Enjoy Your Growth
Journey in the Fascinating Indian Market
it can drive innovation at top-notch speed.Being frugal is not at all bad.
Wahi Anuj
Vice-Chairman & Managing Director
Being frugal is not at all bad, it can drive innovation at top-notch speed.
Anuj Wahi
Vice-Chairman & Managing Director